Epidemiology of colon cancer
Colorectal cancer (CRC) is the second most deadly neoplasm in men and women, with one million new cases per year worldwide. It is also the second most deadly cancer with 500.000 people dying every year worldwide (European Cancer Institute)
CRC is often diagnosed in later stages, drastically worsening prognosis. The disease develops slowly over many years. Most of these cancers start as a polyp, a growth of tissue that starts in the lining and grows into the center of the colon or rectum. Over time, some polyps develop as malignant tumors. It is estimated that only around 30-40% of colon cancers are diagnosed in early stages, and later diagnosis is strongly correlated with poorer prognosis. While cancer diagnosed in early stages (T0-T1) has a 95-100% 5-year survival rate, those diagnosed in medium stages (T2-T3) have a 48-60% survival rate and those diagnosed in advanced stages (T4) have just a 3% survival rate (National Cancer Institute). Early detection programs are essential to decrease mortality around 30-35% and it is more than necessary to develop specific biomarkers for an efficient early diagnosis or prevention.
Limitations in current diagnosis tools
Current available diagnostic tools for CRC show different technical, economical and complexity-related imitations:
- Symptoms appear in late stage so detection based on them leads to a delayed diagnosis.
- Invasive procedures, such as colonoscopy, have good detection capacity but are invasive, complex to perform, costly and potentially can originate serious complications. They are not suitable for frequent population screening. Barium enema is less invasive but its detection capacity is more limited.
- Radiologic diagnosis technologies, such as CT or MRI, offer good detection capacity, but are expensive and complex to use for regular patients screening. Further, CT exposes patients to a non-acceptable amount of radiation for screening purposes. These technologies are suitable for confirmation of suspected pathology.
- Fecal occult blood tests (FOBT) are simple to use and inexpensive. However, their detection capacity is limited. These tests miss a relative large proportion of cancers, mainly at early stages, severely delaying diagnosis in those patients. FOBT tests also falsely identify as potential cancer different benign situations (e.g. polyps) or even normality, forcing to perform unnecessary more complex, invasive and expensive explorations.
- Biomarker genomic tests are non-invasive methods, but with a high cost and limited detection capacity and mainly reflects genetic predisposition instead of proving the disease is present.
- Due to the obvious limitations of these classical detection techniques, is necessary to develop new detection methods in order to overcame the associated inconveniences and, consequently, offer a fast and reliable solution for the clinicians.
Autoantibodies and Tumor Associated Antigens (TAA’s)
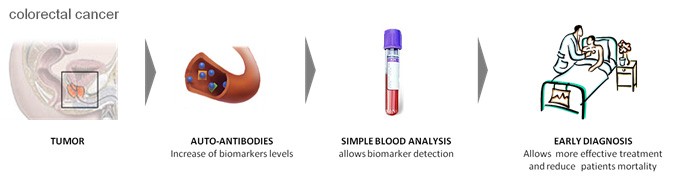
The innovative technological approach of COLODETCT is based on detecting in blood the auto-antibodies generated by human body against proteins related to the colorectal cancer tumor.
An auto.antibody is an antibody (a type of protein) produced by the immune system that is directed against one or more of the individual’s own proteins, such as the tumor antigens present in an cancer.
Tumor-associated antigens (TAA) can derive form any protein or glycoprotein synthesized by the tumor cell. Tumor proteins can be affected by specific point mutations, misfolding, overexpression, aberrant glycosylation, truncation, or aberrant degradation.
Immune system from patients reacts against this TAA’s of the tumor generating several auto-antibodies that can be measured in a simple blood test. The advantage of auto-antibodies as biomarkers is that they appear at detectable levels in patient’s blood early, allowing earlier diagnosis before symptoms are present and even before other types of biomarkers can be measured. Another auto-antibodies feature that makes them suitable for utilization as biomarkers is their high relative concentrations, extended long half-life and good stability in blood.
COLODETECT will simultaneously detect specific autoantibodies (biomarkers) against a reduced number of tumor antigens in serum/plasma samples from CRC patients.